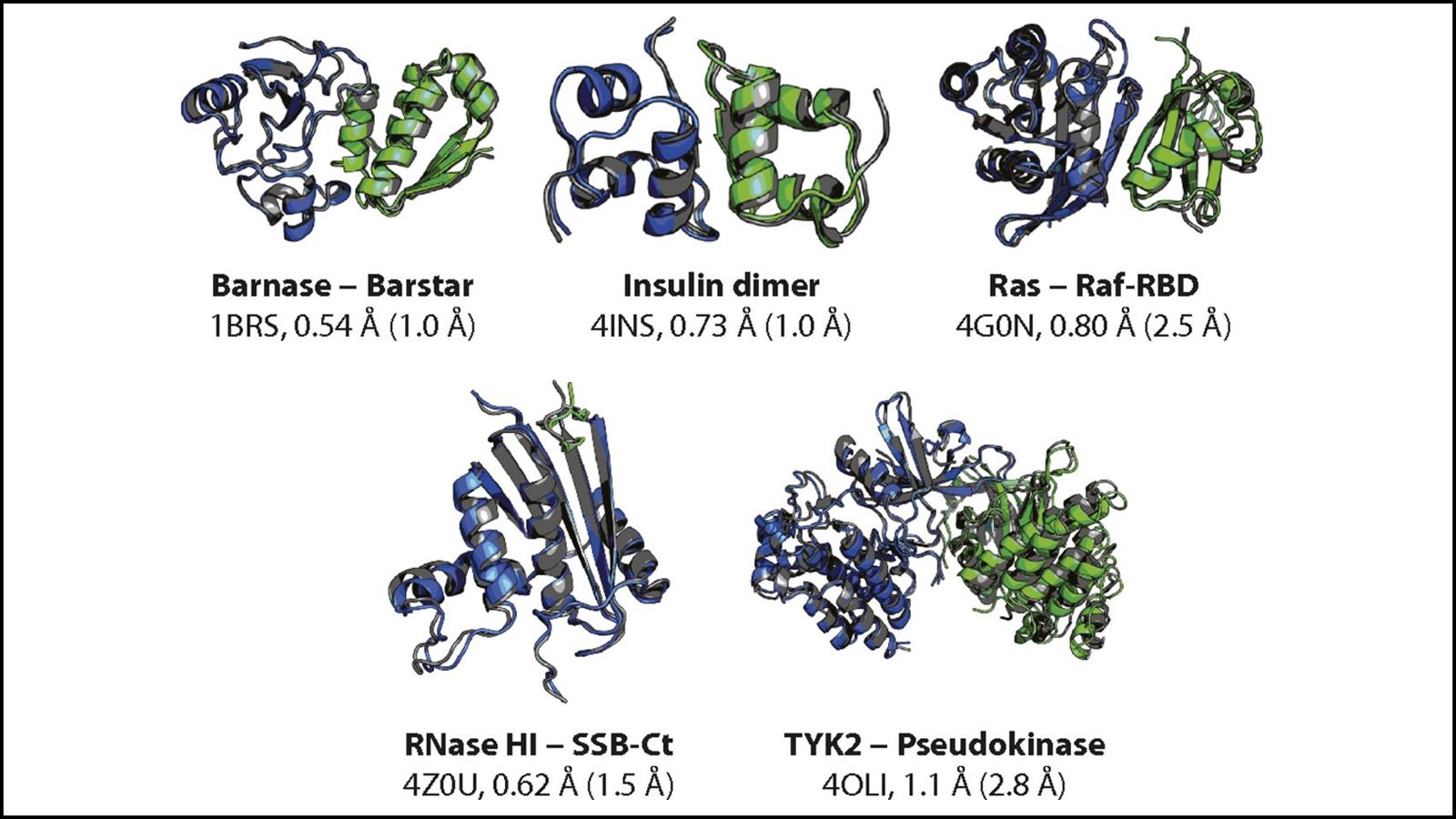
The association of proteins into stable complexes is central to an array of biological processes. Predicting the structures of and understanding the mechanisms by which proteins associate into these complexes are outstanding challenges in the field of molecular biology. Advances to our understanding could also have important implications for drug discovery efforts involving protein-protein complexes, a large and important class of therapeutic targets where progress toward the clinic, especially for small-molecule drugs, has been challenging. To study protein-protein association, we have developed an approach called tempered binding that combines long timescale atomic-level molecular dynamics simulations with simulated Hamiltonian tempering. We will describe the results of tempered binding simulations in which proteins repeatedly associate into, and dissociate from, their crystallographically-determined complex structures, and discuss insights into protein-protein association mechanisms revealed by these simulations.
Hunter College has been alerted to phishing scams targeting the community. Never share Multi-factor Authentication (MFA) codes, passwords, or personal information. Read How to Protect Yourself from Identity Theft.