Reactive A1 Astrocytes Promote Cancer Cell Growth Within the Leptomeninges
| Name | Katie Nikishina |
| Institution | Hunter College |
| Research Field | Basic Cancer Research |
| Role at Institution | Undergraduate Student |
| Presenter(s) | Katie Nikishina |
Weather Alert (Monday, January 26): Hunter may move to remote classes and operations. CUNY/Hunter decision to be made soon. Students should check Brightspace and Hunter email for course-specific instructions from professors. Staff should monitor Hunter email for updates regarding remote work. Read more.
Reactive A1 Astrocytes Promote Cancer Cell Growth Within the Leptomeninges
Katie Nikishina, Yudan Chi, PhD and Adrienne Boire, MD, PhD
Human Oncology & Pathogenesis Program and Department of Neurology
Memorial Sloan Kettering Cancer Center NY, NY
Scientific Discipline 2 Cancer Biology, a Cancer Biology
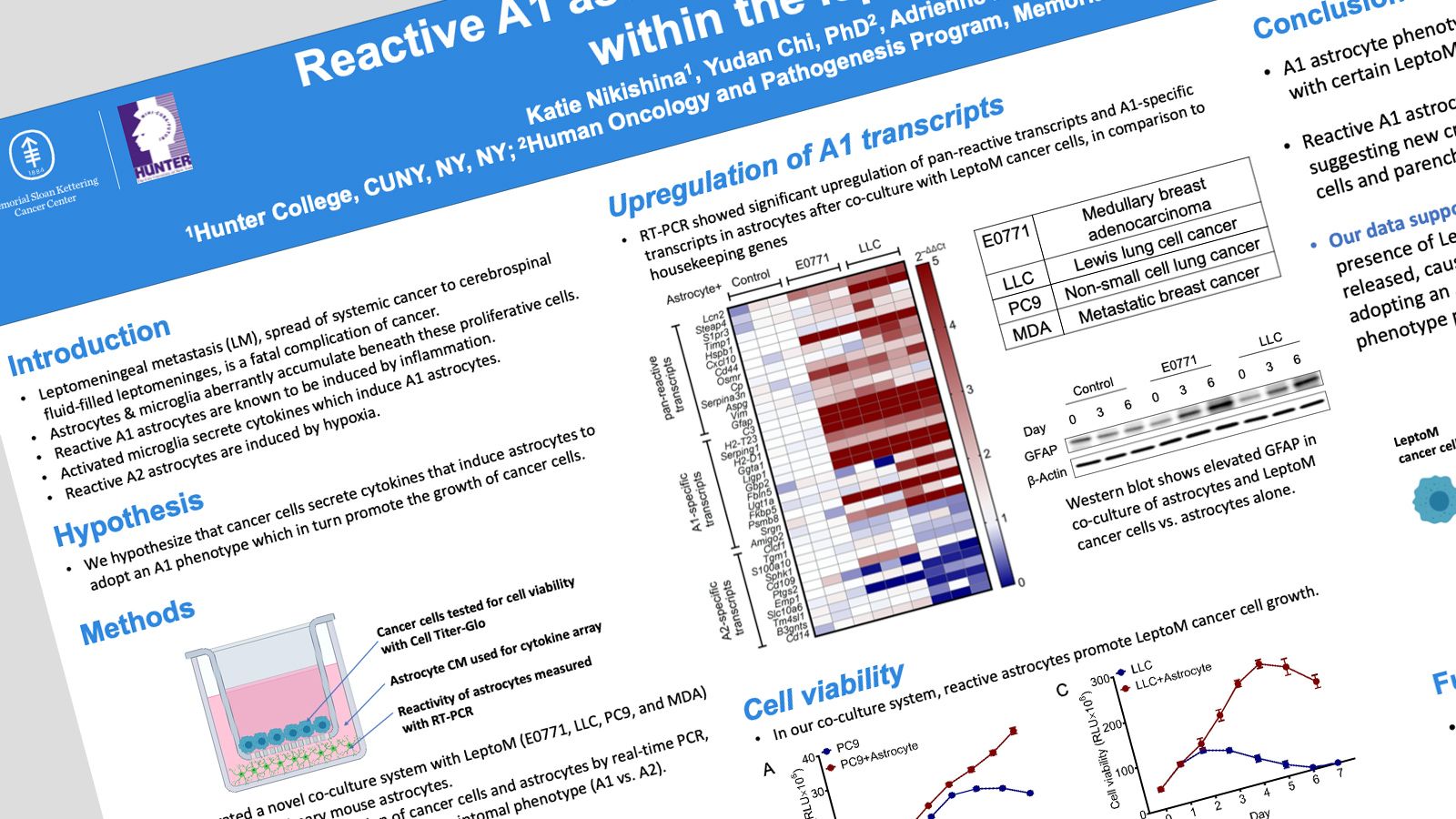
Leptomeningeal metastasis (LM), spread of systemic cancer into the cerebrospinal fluid-filled leptomeninges, is a fatal complication of cancer. Within the leptomeninges, cancer cells may float within the spinal fluid or adhere to the parenchyma. We observe proliferative adherent cancer cells in mouse models of LM. Beneath these proliferative cells, we observe aberrant accumulation of microglia and astrocytes. Others have shown that reactive astrocytes are induced by neuroinflammatory microglia secreting Il-1𝞪;;, TNF𝞪;;, and C1q cytokines. CNS injury is known to induce reactive astrocytes (A1) which may contribute to neuron death and axotomy. We hypothesize that cancer cells will secrete cytokines that induce astrocytes to adopt an A1 phenotype, resulting in the death of neighboring neurons and oligodendrocytes and proliferation of cancer cells. We measured cancer cell proliferation in vivo by immunohistochemistry (IHC) of formalin-fixed, paraffin embedded brains from our LM mouse models. To investigate the relationship between cancer cells and astrocytes, we generated a novel co-culture system with Leptomeningeal Metastatic (LeptoM) cancer cells (LLC, E0771, MDA or PC9 model systems) and primary mouse astrocytes. Gene transcription of the cancer cells and astrocytes was measured by real-time PCR (RT-PCR), allowing us to identify astrocyte transcriptomal phenotype (A1 vs. A2). Cancer cell growth was measured by Cell Titer Glo assay. IHC staining demonstrated that cancer cells adherent to the leptomeninges maintain high proliferative capacity. RT-PCR revealed significant upregulation of pan-reactive transcripts and A1-specific transcripts in astrocytes after co-culture with LeptoM cancer cells, in comparison to housekeeping genes. After three days of co-culture, LLC, E0771, and astrocytes demonstrated increased cancer cell viability compared with cancer cells alone. Our preliminary findings demonstrate that the A1 astrocyte phenotype is induced by co-culture with certain cancer cells. In addition, A1 signaling from astrocytes promotes cancer cell growth, suggesting new crosstalk between cancer cells in the leptomeninges and parenchymal astrocytes.
Email questions and comments about this abstract to Katie.Nikishina33@myhunter.cuny.edu.
