



The BioImaging Facility at Hunter College is centered in a multi-room facility of 1024 sq. ft. located in the Biological Sciences Department on the 8th Floor of Hunter North building. A satellite facility also includes a number of instruments on the 4th Floor of the Belfer Research building (at 69th Street and York Ave). Faculty and students have access to a broad spectrum of instruments, ranging from simple white light wide-field microscopes to fluorescent multidimensional super-resolution and confocal imaging systems. The Faculty supervisor and Scientific Director is Dr. Diana P. Bratu. Dr. Lloyd Williams is the Managing Director of the facility. The facility staff has expertise in many areas of microscopy including the laser scanning confocal microscopy, super-resolution microscopy, two-photon microscopy. They are also familiar with many image analysis software packages, including, Imaris, Volocity, Autoquant, MetaMorph, and NIS-Elements. Detailed descriptions of the equipment in the facility is given below. All equipment is located at Rm 826 HN or at the 4th floor of the Belfer Research Building where designated.
To book time on any of the instruments go to http://bookit.hunter.cuny.edu

The Nikon TIRF SIM microscope allows the users to do both Total Internal Reflection Microscopy and SIM super-resolution microscopy. The acquisition software is Nikon NIS-Elements.
This machine is in 826HN
The charge for this instrument is $20/hr.

The Nikon A1 Confocal microscope is Nikon's powerful fully-automated confocal imaging system, capable of capturing high-quality confocal images of cells and molecular events at high speed and enhanced sensitivity. The acquisition software is NIS-Elements. The system is located at Belfer Research Building.
The charge for this instrument is $20/hr.

The SLIDEVIEW™ VS200 research slide scanner enables you to capture high-resolution images of slides for quantitative analysis.
The charge for this instrument is $20/hr.

The Nikon Eclipse Ti scope is a wide-field fluorescent microscope. It is equipped with Andor iXon EMCCD camera and a DG5 wavelength switcher. It is also equipped with an Andor Mosaic/MicroPoint system for Optogenetics, Opto physiology, photobleaching/activation and uncaging applications
This machine is in 826HN
The charge for this instrument is $15/hr.

The UltraView is a spinning disk confocal microscope equipped with five laser lines, which allow visualization of GFP, RFP, CY5, DAPI, CFP, and YFP, etc. The UltraView ERS system allows for high-speed, multiple-probe, time-lapse experiments; NIS-Elements software is used for image acquisition and analysis.
This machine is in 830HN
The charge for this machine is $20/hr

The Leica TCS SP8 DLS is a dual function fluorescence microscope that can be used as a conventional laser scanning confocal microscope (LSCM) or as a lightsheet fluorescence microscope (LSFM).
This machine is in 830HN
The charge for this instrument is $20/hr.

The TCS SP2 Laser Scanning Spectral Confocal Microscope can do measurements of transmitted light, fluorescence and laser scanning fluorescence imaging.
This machine is in 826HN
The charge for this instrument is $20/hr.

The calcium ratio imaging system consists of: a Nikon Eclipse TE 200 inverted epifluorescence microscope, Sutter Lambda 10-2 optical filter system, and Nikon NIS-Elements imaging software with Calcium & FRET plug-in. The system also is equipped with a Narishige micromanipulator system.
This machine is in 826HN
The charge for this instrument is $10/hr.

The Nikon Ti-S microscope has a SOLA Light Engine solid state light source and a Nikon DigiSight camera. It has filter sets for DAPI FITC and RFP
The charge for this instrument is $5/hr.

JEOL JEM-100CX II transmission electron microscope is an advanced high-performance electron microscope with stable and excellent imaging capabilities at low to high magnifications (90X to 800, 000X). A 10M-pixel HAMAMATSU C4742-95 digital camera is integrated into the system for high-resolution image acquisition.
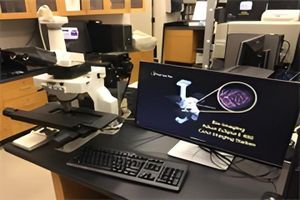
The Nikon Color Imaging system consists of a Nikon Eclipse E400 upright microscope, and Nikon DXM 1200F high-resolution (3,840 x 3,072 pixels) color video camera. The microscope is equipped for fluorescence and has phase contrast and Nomarski optics. The system is interfaced to a 1.4Ghz PC which utilizes Nikon Imaging Software. The system also has Adobe Photoshop installed for image acquisition and manipulation
The charge for this instrument is $5/hr.

The Imaris Imaging station is a high-power workstation with Bitplane's Imaris Imaging software installed. Imaris provides functionality for the visualization, segmentation, and interpretation of 3D and 4D microscopy datasets.
the charge for this instrument is $10/hr.

This Imaging workstation is a high-power workstation with Nikon's NIS-Elements Imaging software installed. NIS-Elements provides cutting edges tools for image manipulation and data management. It also has Imaris 9.12 installed
The charge for these instruments is $5/hr for Elements and $10 per hour for Imaris.

This Imaging workstation has both AutoQuant and Nikon's NIS-Elements Imaging software installed.
AutoQuant is used to deconvolve images acquired in the facility.
This machine also has a floating license of Imaris 9.6
The charge for this instruments is $5/hr for Elements and and $10 for Imaris and AutoQuant.

This Imaging workstation has Nikon's NIS-Elements Imaging software installed. Additionally, it has Element's deconvolution module installed. This machine also has a floating license of Imaris 9.6
The charge for this instruments is $5/hr for Elements and and $10 for Imaris.

The Molecular Devices SpectraMax Gemini EM Microplate Spectrofluorometer features top and bottom reading optics, dual monochromators, wavelength scanning, well scanning, auto PMT gain and is driven by Softmax Pro software on a Windows-based controller.
The charge for this instrument is $5/scan.

Typhoon is a highly sensitive variable-mode gel imager. The Typhoon 9410 unites the ability to detect an extensive variety of fluors, with proven storage phosphor autoradiography technology and direct imaging of chemiluminescence. The typhoon can also be used to analyze microarrays.
The charge for this instrument is $5/scan.

Typhoon FLA 7000 is a fast laser scanner for biomolecular imaging applications including sensitive and quantitative measurements of radioisotopic labels, chemifluorescent Western blots, and single fluorescence.
The charge for this instrument is $5/scan.

The Odyssey replaces the tradition methods of analyzing western blots, chemiluminescence, and fluorescence detection, with near-infrared (NIR) fluorescence detection. This allows you to quantitate proteins over a much wider linear dynamic range than chemiluminescence can. This feature can enable strong and weak bands on the same blot to be accurately quantified without the need for multiple exposures. In addition, the Odyssey is uniquely equipped with two infrared channels 700 nm and 800 nm, and can thus probe two different targets in the same experiment.
The charge for this instrument is $5/scan.

PowerWave HT is a multi-channel reader for maximum speed in both 96- and 384-well plate formats. The PowerWave HT provides flexibility for multiple applications, including endpoint, kinetic and spectral scanning mode. Powerful Gen5 PC-based software is used for system control and data analysis.
The charge for this instrument is $3/scan.

Synergy HTX is a Multi-Mode Microplate Reader for making: absorbance, fluorescence, luminescence and AlphaScreen/AlphaLISA measurements on 6- to 384-well microplates.
This instrument is in room BB 453
The charge for this instrument is $3/scan.

The GloMax®-96 Microplate Luminometer is a state-of-the-art Microplate Luminometer with a high sensitivity and broad dynamic range that is necessary for chemiluminescent and bioluminescent applications. The GloMax uses an advanced photon-counting photomultiplier tube (PMT), with a dynamic range of greater than nine logs. This range covers virtually all chemiluminescent and bioluminescent assays, eliminating the need to dilute samples or manage detector-driven gain changes.
The charge for this instrument is $5/scan.

The Leica CM 3050S Cryostat features motorized sectioning and programmable defrost cycles. The cryostat can cut sections in the range 0.5 to 300 mm. The max specimen size is 55 X 70 mm and can cool samples down to - 50°C.
The charge for this instrument is $5/hr. Sign the log book when you use this system
To book time on this system use the Cryostat SharePoint Calendar at

The facility has a floating Imaris license that can be installed on certain machines on the 8th and 9th floor of Hunter North. The charge for using this license is $10 per hour.
We also have satellite license that can be issued for off-campus use.
The charge for this is $10 for a day or $40 for a week.
The facility charges $20 per hour for use of this microscope. There is a $20 minimum charge, and fractions of an hour count as whole hours. For long time duration experiment, we have a special rate policy described as follows: in any 24 hour period
Email Lloyd Williams ( williams@genectr.hunter.cuny.edu) in advance for applying this policy.
The facility charges $20 per hour for use of the confocal. There is a $20 minimum charge, and fractions of an hour count as whole hours.
The facility charges $20 per hour for use of the confocal. There is a $20 minimum charge, and fractions of an hour count as whole hours.
The facility charges $20 per hour for use of this microscope. There is a $20 minimum charge, and fractions of an hour count as whole hours.
The facility charges $15 per hour for use of this microscope. There is a $15 minimum charge, and fractions of an hour count as whole hours.
The facility charges $10 per hour for use of the microscope. There is a $10 minimum charge, and fractions of an hour count as whole hours.
The facility charges $5 per hour for use of the Cryostat. There is a $5 minimum charge, and fractions of an hour count as whole hours. Please sign the log book.
The facility charges $5 per scan. Use is monitored by the event log on the computer attached to the machines.
The facility charges $3 per scan. Use is monitored by the event log on the computer attached to the machines.
The facility charges $5 per hour for use of this image analysis package. There is a $5 minimum charge, and fractions of an hour count as whole hours.
The facility charges $10 per hour for use of this image analysis package. There is a $10 minimum charge, and fractions of an hour count as whole hours.
The facility charges $10 per hour for use of this image analysis package. There is a $10 minimum charge, and fractions of an hour count as whole hours. The charge for use of the core facilities version of Imaris is also $10 /hour. Satellite license use is $10 per day or $40 per week.
Nowadays, microscopic imaging techniques are becoming more and more popular in biomedical research. Most of the advanced high-end microscope systems (i.e., confocal & spinning disk systems) are expensive, not every research lab can have enough fund to support such system for their research. A solution to overcome this problem is to share the microscope systems through the Internet (also called remote instrumentation): remote users can get the control of the microscope for their experiment through a simple Internet connection. Our approach for this remote instrumentation task is to combine the powers of WebEx and PVX:
Utilize WebEx to setup remote desktop sharing for microscope control.
Utilize PVX monitoring system to setup Internet video conferencing for remote communication purpose.
A new service is ready for our remote users to get remote access to our advanced confocal microscopes for their own imaging purposes.
Our service includes:
For scheduling the above remote instrumentation service, please check the following guidelines:
| Glomax 96 Microplate Luminometer |
Biotek PowerWave HT Plate Reader |
SpectraMax Gemini EM Fluorescence Spectrometer |
Typhoon 9410 Imager | LI-COR Odyssey | |
|---|---|---|---|---|---|
| Sample Type | 96-well plate | 96 & 384-well plate | 96 to 384-well plate | Plate/Gel/Blot | Western blot sample |
| Excitation Wavelength Range |
N/A | 200-999nm | 250-850nm | 457nm/488nm/ 532nm/633nm |
680nm/780nm |
| Detection Wavelength Range |
300-650nm | 200-999nm | 360-850nm | 500-685nm | Near-infrared detection 680-1000nm |
| Detection Mode | Luminescence | Absorbance | Fluorescence | Phosphorimaging Chemiluminescence Fluorescence | Fluorescence |
| Read Mode | Endpoint/Kinetics | Endpoint/Kinetics | Endpoint/Kinetics /Spectrum |
Image | Image |
| Application | Chemiluminescence Bioluminescent assay | Direct DNA quantitation Purity testing RNA quantitation ELISA Enzyme Kinetics Colorimetric assays |
ELISAs and Immunoassays Nucleic Acid Quantitation Protein Quantitation Reporter Gene Assays Cell Viability, Proliferation, and Cytotoxicity Enzyme Assays Transporter Assays Phosphatases/Kinases Microbial Growth |
Quantitative Phosphorimaging ECL Plus Westerns Multifluorescence applications (such as 2-D DIGE and ECL Plex) | Quantitative Western ELISA/FLISA In-cell Western Assay In-Gel Western Assay On-cell Western Assay Microwestern |
| LensSystem | Nikon Upright | Nikon Inverted | Leica Confocal | PE Spinning-disk | Nikon TIRF/SIM | Nikon A1 | Leica SP8 |
|---|---|---|---|---|---|---|---|
| Objective Description Magnification/NA /Immersion (dry lens unless mentioned) |
4x/0.13 | 4x/0.1 | 5x/0.15 | 20x/0.45/multi | 40x/0.6 | 100x/1.45/oil | 100x/1.40/oil HC PL APO |
| 10x/0.45 | 10x/0.3 | 10x/0.4 | 60x/1.49/oil | 60x/1.49/oil | 60x/1.45/oil | 63x/1.40/oil HC PL APO | |
| 20x/0.75 | 20x/0.45 | 20x/0.5 | 100x/1.4/oil | 100x/1.49/oil | 40x/1.25/oil | 40x/1.3/oil HC PL APO | |
| 40x/1.0/oil | 40x/0.6 | 40x/1.25/oil | 40x/1.3/oil | 20x/0.75 | 10x/0.40 HC PL APO | ||
| 60x/1.4/oil | 63x/1.4/oil | 10x/0.45 | 5x/0.15 HC PL FLUOTAR | ||||
| 100x/1.4/oil | 2.5x/0.007 HC PL FLUOTAR |
The Leica SP8 also has the following DLS objectives 1.6x/0.05, 5x/0.15, 10x/0.3, 25x/0.95
and DLS TwinFlect 2.5mm, 5mm, 7.8 mm(water) 7.8mm (glycerin)
Notice: all instruments are in HN826, except where noted.
| Instrument | Laser | Wavelengths |
| Leica SP2 Confocal | Argon Ion Laser | 458, 476, 488, 514 nm |
| Leica SP2 Confocal | Melles Griot Solid State Laser | 561 nm |
| Leica SP2 Confocal | HeNe Laser | 633 nm |
| PE Spinning-disk | Solid State Laser | 561 nm |
| PE Spinning-disk | Solid State Laser | 405 nm |
| PE Spinning-disk | Solid State Laser | 488 nm |
| PE Spinning-disk | Solid State Laser | 514 nm |
| PE Spinning-disk | Solid State Laser | 440 nm |
| PE Spinning-disk | Solid State Laser | 640 nm |
| Nikon TIRF/SIM - TIRF Module | Solid State Laser | 405 nm |
| Nikon TIRF/SIM - TIRF Module | Solid State Laser | 488 nm |
| Nikon TIRF/SIM - TIRF Module | Solid State Laser | 561 nm |
| Nikon TIRF/SIM - TIRF Module | Solid State Laser | 640 nm |
| Nikon TIRF/SIM - SIM module | Solid State Laser | 488 nm |
| Nikon TIRF/SIM - SIM module | Solid State Laser | 561 nm |
| Nikon TIRF/SIM - SIM module | Solid State Laser | 640 nm |
| Nikon Eclipse Ti Mosaic/MicroPoint System & FRAP | Solid State Laser | 405 nm |
| Amersham Biosciences Typhoon 9410 | Argon Ion Laser | 457, 488 nm |
| Amersham Biosciences Typhoon 9410 | SYAG laser | 561 nm |
| Amersham Biosciences Typhoon 9410 | HeNe Laser | 640 nm |
| Nikon A1R Resonant Confocal ( Room Belfer BB 479) | Solid State Laser | 405 nm |
| Nikon A1R Resonant Confocal ( Room Belfer BB 479) | Solid State Laser | 488 nm |
| Nikon A1R Resonant Confocal ( Room Belfer BB 479) | Solid State Laser | 561 nm |
| Nikon A1R Resonant Confocal ( Room Belfer BB 479) | Solid State Laser | 640 nm |
| GE FLA 7000 Typhoon (Room Belfer BB ) | Solid State Laser | 473 nm |
| GE FLA 7000 Typhoon (Room Belfer BB ) | Solid State Laser | 532 nm |
| GE FLA 7000 Typhoon (Room Belfer BB ) | Solid State Laser | 635 nm |
| GE FLA 7000 Typhoon (Room Belfer BB ) | Solid State Laser | 650 nm |
